Are you searching for the Labeled Periodic Table of elements with the name? We have searched for the periodic table or P table on the internet but do you know; we did not get complete information related to the table. You did not need to worry as you will get every piece of information about the periodic table on our website.
Related Article Here:-
- Oxygen Valency Electrons
- Bromine Electrons Configuration
- Blank Periodic Table Element
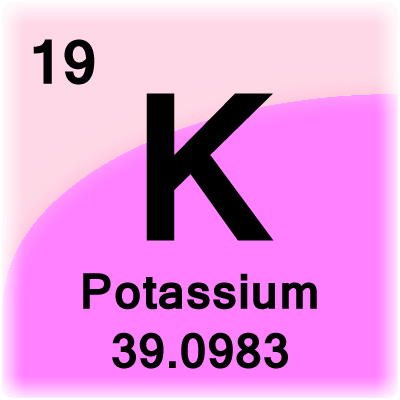
- Potassium Periodic Table
- Labelled Periodic Table
- Electronegativity Chart
- Lead Electron Configuration
- Oxygen Valence Electrons
You will also get the lists of Labeled Periodic Tables so you can download them of your choice. We have one of the most advanced periodic tables that is HD periodic table. It will give you a clear view and information about metals and elements. You can view it on your mobile, laptop, or tablet.
You will get the name of elements with the increase of the atomic number. Every element has its unique symbols, which consist of one or two alphabet letters. The element number, which is also known as atomic number, is nothing but the protons of the elements.
For Example:
- A – Hydrogen
- He – HELIUM
- Li – Lithium
- Be – Beryllium
- C – Carbon
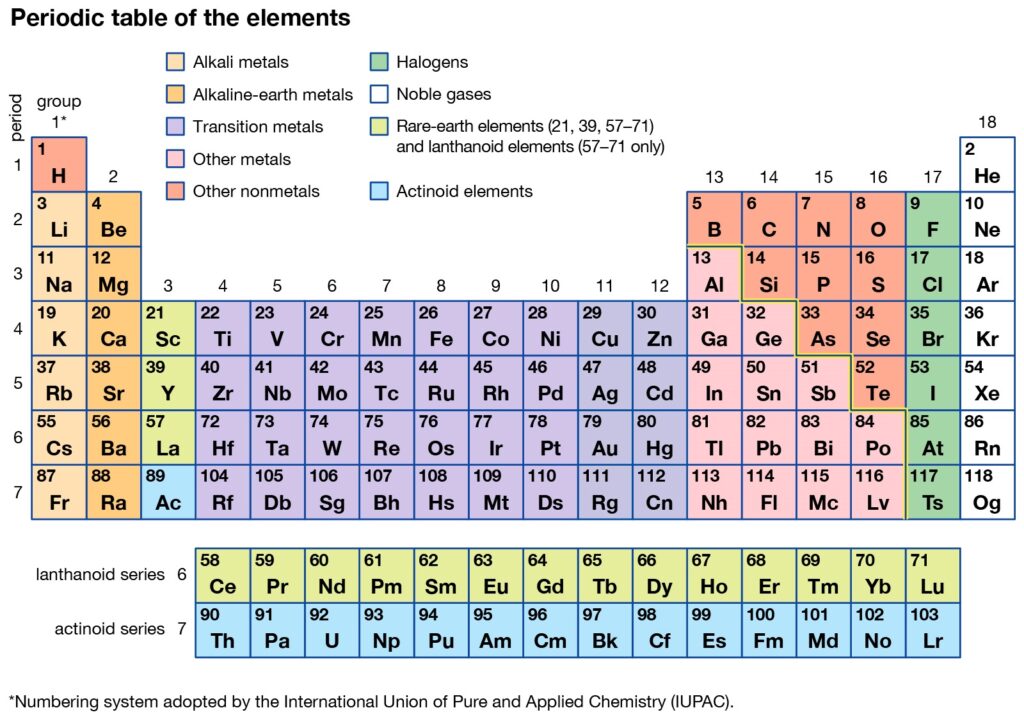
Labelled Periodic Table of Element
You can find the periodic table in many chemistry books. The tables in every book are different because every book wants to give you some information differently. The different ways of learning help you to learn something new. Do you know, we have chemistry in every part of our life.

The food we eat, the water we drink, the cooking process, the digestion process, the excretion process, etc., every step needs chemistry and chemical reaction. So, to know these things in detail, the first and essential thing you need to know is the periodic table. The periodic learning table starts from the class 5th in almost every school.
When you are going to solve a chemical equation, then you will need this periodic table. The students or the scientist always keeps the periodic table for reference purpose. You can also use it for personal purposes, i.e., for learning something new. The kids learn the element’s name, atomic mass, neutrons, protons, electrons, etc. They learn organic and inorganic chemistry.
Labelled Periodic Table Metals
You will get the perfect Labeled Periodic Table that you need. We have a complete collection of the free periodic table in excel, PDF, and PNG images so, and you only need to download, print, and use it. This chart gives you complete information like electronegativity, ionization energy, metallic character, electrons affinity, and atomic radius.

We also notice the noble gases present on the right-hand side of the periodic table. The periodic table is helpful for those students who are going to appear in a competitive exam, whether it is neet, jee mains, or any job exam. So, if you want to get higher marks in the competitive exam, you will need this. We also have a blank periodic table for the students.
Periodic Table Labeled Groups
There are 118 elements in the periodic table, out of which 94 elements are natural, and others are nuclear reactor or laboratory tested elements. There are 18 groups in the periodic table, which consists of metal and nonmetal. Protons in the tables are positively charged particles. Neutrons are the neutrally negative charge, and electrons are the negative charge particles. It also shows the formation of a bond from one element to the other.

Labelled Periodic Table with Charges
The periodic table with the charges has information about the positive and negative charges on the atom. You did not need to search a lot for the perfect periodic table as we have a black and white, colourful, dynamic, blank, Hd periodic table.

There is a separate downloading link for each type of table, so you only need to tap on the downloading link of your favorite periodic table, and you will have it on your device. We also have printable formats of the periodic table, so you can also take the printout and use it anywhere.