Get to learn the Silicon Electron Configuration and develop the proper understanding of this chemical element. As we progress with the article, we shall make our discussion about the electron configuration and the orbital movement of this particular chemical element. We can assure you of the fact that you are going to explore some significant details about this chemical element for your academics and professional usage as well.
![]()
In chemistry, Silicon is one of the most renowned chemical elements that comes with its atomic number of 14 and the symbol of Si. The element in its general appearance looks like a bristle metallic element that belongs to group 14 in the periodic table. The thing with silicon is that it’s one of the most common elements in the universe; however, its quality is more available in space than on Earth.
Related Article:-
- Oxygen Valence Electrons
- Bromine Electron Configuration
- Blank Periodic Table Element
- Potassium Periodic Table
- Labeled Periodic Table
- Electronegativity Chart
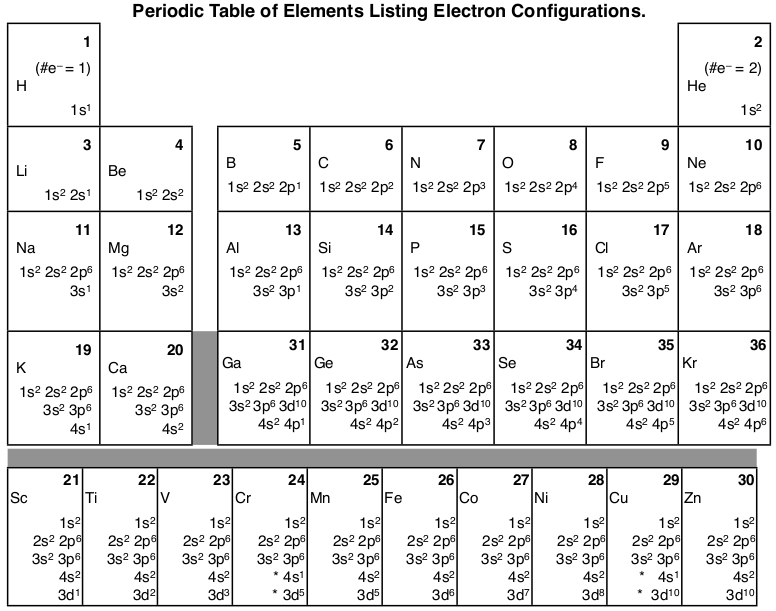
Silicon Electron Configuration
Well, the Silicon Electron Configuration is one of its most significant chemical properties that scholars need to understand. The electron configuration is the process by which a chemical element distributes its electrons into its orbitals.
This process is integral for all types of chemical elements in chemistry and displays some significant features of the element. So, in the case of Silicon, we have its electron configuration is [Ne] 3s2 3p2, which you can learn for your academics or the formal requirements.
Electron Configuration For Silicon Ion
The Silicon Electron Configuration ion is the specific state of the chemical element, which represents the electric charge state of the element when it loses or gains one more electron. So, as a part of its chemical properties, you should be aware of its electron configuration, which we are going to discuss here.
The electron configuration for the ion state of Silicon is [Ne] 3s² 3p², which comes from its periodic table. You can learn it as part of the chemical’s integral properties to use it for your academics or professional uses as well.
Silicon Number of Valence Electrons
The valence electrons or the valency of any chemical element is the same thing that we mostly refer to. The valence electrons are the number of electrons that are present in the outer shell of the element. We often term valency as the combining capacity of Silicon with the other elements.
The numbers of valence electrons of Silicon Electron Configuration (1s22s22p63s23p2) always play a huge role in the combining capacity. With silicon, we have the 4 valence electrons that become the valence of this element.
Ground State Electron Configuration For Silicon
Well, the ground-state electron configuration of any chemical element is used in terms of different terminology. It depicts the arrangement of Silicon electrons around the nucleus of the atoms. In this process, the electrons from the orbitals that possess the variable energy fall to the lower energy state.
This state is generally referred to as the ground state, and we calculate the ground state electron configuration of silicon. Knowing the ground-state electron configuration of this element will ensure you know its other dimensions.
What is the Electron Configuration of Silicon
Well, the electron configuration of Silicon represents one of the most significant chemical properties of the element. It is the process in which the electrons of the elements take their precise position in their orbitals. Subsequently, we end up getting the equation that helps us in understanding the ultimate pattern of electron distribution of the element.
In the case of Silicon, that equation of electron configuration turns out to be [Ne] 3s² 3p². There are further implications of the electron configuration of the element that are useful in establishing its other properties. For instance, with the electron configuration, we tend to get the ultimate and fixed standing of the element in its periodic table for all scholars.
How Many Valence Electrons are in Silicon
The valency of any chemical element depends on the valence electrons that the element has. These valence electrons are the part of the electrons that remain in the outer shell of the element.
These valence electrons of the Electron Configuration are significant as they contribute directly to the combining process of the element with other elements. Silicon has the number of 4 valence electrons that remain present in its outer shell, and is a significant aspect of it.
Silicon Orbital Diagram
Well, the orbital diagram of Electron Configuration is the best source of information if you are willing to study the chemical bonding of silicon. This is a kind of qualitative diagram that explains the whole combining process of the element in the best possible manner.
With this diagram, one can also explore the pictorial representation of the electrons and where they go in the orbital. In conclusion, the orbital diagram of silicon presents the bigger picture of silicon along with its most significant chemical properties.