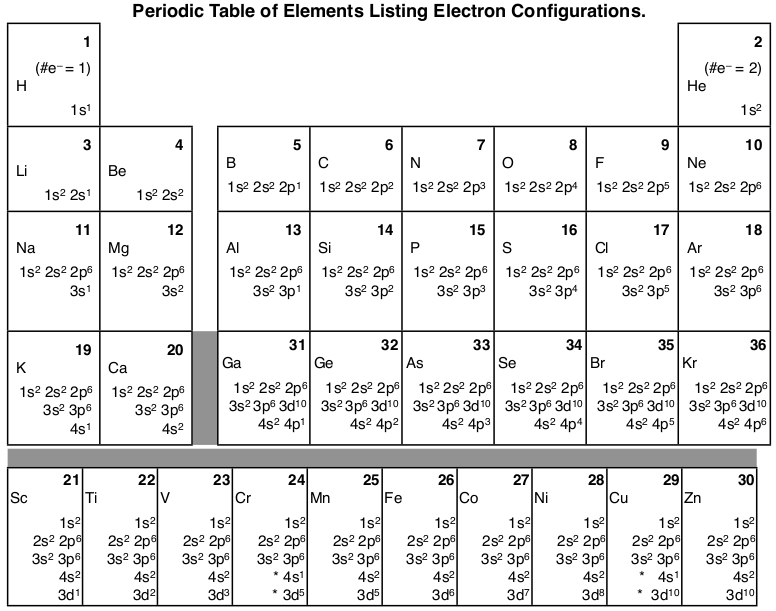
Electron Configuration Chart: Today, through the use of our article, the users are going to know about certain terms of chemistry and without which the users or anyone cannot master the subject.
Therefore, if you are new to this subject and are finding difficulties in solving questions related to electronic configuration, then you have landed at the right place, as today, while you leave from here, you will be able to clear at least some of your concepts.
- Oxygen Valence Electrons
- Bromine Electron Configuration
- Blank Periodic Table Element
- Labelled Periodic Table
- Electronegativity Chart
What is Electron Configuration?
Firstly, for new users or students, they must keep practising the topic because chemistry is similar to a subject like maths. Just like maths needs proper practice and time, similarly, chemistry also needs proper time and practice from time to time. One cannot be perfect in this subject in a day or two, but to make it one of your favourite subjects, one has to study and keep revising the topic again and again.
When we talk about the term Electron Configuration Chart, in short words, it shows the exact distribution of various elements in their respective atomic orbitals. Every element has its electronic configuration,n, and no two elements can have the same.
Electron Configuration Diagram of All Elements
Now that you have come to know what is meant by Electron Configuration Chart, the main use of the electronic configuration is in finding the valence of any or various elements. Apart from that, it also helps in forecasting certain properties of the elements that are present in the groups.
One must also know that the elements whose Electron Configuration Chart is similar to each other also exhibit similar properties. One must have heard about quantum chemistry, so the electronic configuration plays a major role in quantum chemistry.
One must have also come through the term, which is named as shells and their subshells. So one doesn’t have to get confused as much as one is a part of the other. Shell is the gathering of the subshells with its principal quantum number (is the same). If we say in short, then without a subshell, the shell cannot be found, and similarly, without a shell, one cannot have its subshells.
Subshells are also similar to the shells; the only difference is that it is the gathering of orbitals with the same principal quantum number and angular momentum. One major point is that the subshells in which the electrons are divided are completely based on an azimuthal quantum number. The symbol of the term azimuthal quantum number is denoted by “I”.
- Lead Electron Configuration
- Vanadium Electron Configuration
- Nitrogen Electron Configuration
- Boron Electron Configuration
- Cesium Electron Configuration
- Strontium Electron Configuration
- Carbon Electron Configuration
Electron Configuration Worksheet
Now, many of the users must be wondering why they are now reading the content, but how is it possible that they can use and refer to it anytime they want?. So now is the solution and also a good opportunity for you guys, as we are coming up with our new worksheet, which will have the various electronic configurations of the elements.
In today’s world, everything is almost chargeable, and due to this reason, many users cannot afford some things which are related to education., Today, the fees of various private tuitions and coaching centres are so much that few students can even think of joining. So to make it easier, we have come up with that solution, and that is our worksheet is available free of cost, and no charges are needed to use our worksheet.
The users can refer to our worksheet can download the worksheet, and will be able to use our worksheet whenever they want. They can get the hard copy of the worksheet too by going to any nearby printing shop and can get the printable form of it. The worksheet will have all the Electron Configuration charts of every element, and through our sheet, they can clear all their doubts and queries and can use it any number times.
Electron Configuration Chart
Before you come to the part of electronic configuration, you must come to know about the various elements of the periodic table. If you are looking for a periodic table along with its electronic configuration, then you can find it in our worksheet, as we have mentioned above. The worksheet has all the electronic configurations of every element. Firstly, the users must know how many elements are in the periodic table.
For those users who don’t know how many elements are there, so for them, the answer is that there are 118 elements mentioned in the periodic table. It is not very easy to remember all the elements, but those students who are new to this subject can learn the first 20 elements as they reach their higher level class or grade, and they will be able to manage the first 20 elements.
Apart from that, it is also important to know the full Electron Configuration Chart of those elements, and to get the full information of any elements, we have mentioned our worksheet, and they can get the full information there onwards. The users can master the topic in very few days only through the sheet, and the most important thing about our worksheet is that it is open to all age groups. Usually, many users find difficulties in entering the information as it requires a certain age limit.
How To Do The Electron Configuration?
Many would have come across that if you learn a particular topic in the morning, so if you try to remember it in the evening, you will surely not remember the full concept; therefore, it is important that you have to keep revising the topic again and again. Not only by reading it by doing it practically, is more important that you get to the depth of it.
Various things are too important to find the Electron Configuration Chart, and that is that firstly, it is important to know the atomic number of the element (without this, one cannot find the electronic configuration). Once you get to know the atomic number, the next step is to find the charge of the element. Once you get to know the charge, you need to understand the notation of the element and the valence of that element. Once you are done with the shells, you can put them in their respective orbitals.
It is not a very difficult task to find the Electron Configuration Chart of the element. What only matters is how much time and devotion you are giving to the subject, and whether it be this subject or any other, one has to clear their doubt, and then only it will be easier for them to master the subject.